Yes, exercise can make cancer patients live longer.
A recent study of bowel cancer patients showed that people who had a “structured exercise program” had better fitness and a longer life.
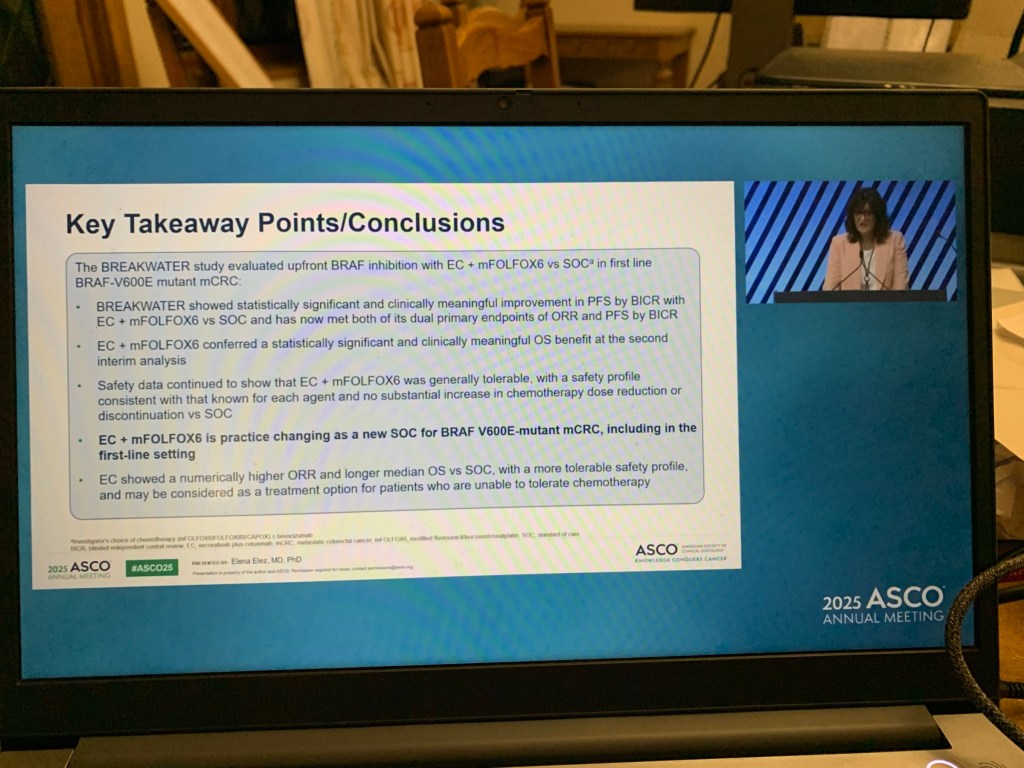
The study data was presented at the prestigious annual meeting of the American Society of Clinical Oncology (ASCO) at Chicago.
Dr Sundar is a member of American Society of Clinical Oncology (ASCO) and has been a member for more than 25 years.
References
- ASCO abstract. A randomized phase III trial of the impact of a structured exercise program on disease-free survival (DFS) in stage 3 or high-risk stage 2 colon cancer: Canadian Cancer Trials Group (CCTG) CO.21 (CHALLENGE).
- CNN news. Exercise may help patients with colon cancer live as long as those who never had it, study suggests
By Madeline Holcombe, CNN
4 minute read
Published 3:02 PM EST, Mon February 24, 2025 - CNN. New research presents promising findings on colorectal cancer treatment and prevention
- BBC news. Major study shows exercise improves cancer survival.

Disclaimer: Please note – This blog is NOT medical advice. This blog is NOT a expert medical opinion on various topics. This blog is purely for information research only and do check the sources where cited. Please DO consult your own doctor to discuss concerns and options, which are relevant and specific to you. The views expressed in this blog are NOT, in any way whatsoever, intended to be a substitute for professional advice. The blog is NOT previewed, commissioned or otherwise endorsed, in any way, by any organisation that the author is associated with. The views expressed in this blog likely represents some of the author’s personal views held at the time of drafting the blog and MAY CHANGE overtime, particularly when new evidence comes to light.