Multiple new drugs have been developed lung and breast cancers over the last decade.
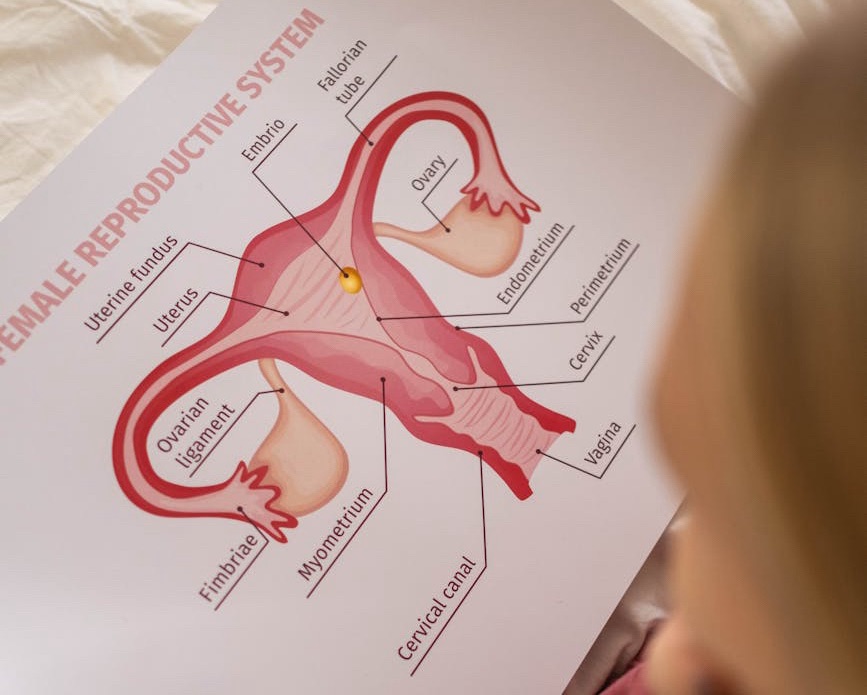
But only a few new drugs have been approved for ovarian cancers in the past decade.
Drugs like Olaparib and Niraparib – which have been approved for ovarian cancers recently – are maintenance drugs used after chemotherapy rather than used as an upfront treatment .
So it is very good news that a brand new drug is likely to enter the market in the near future.
The new drug is called Relacorilant.(Rela).
It has been tested in ovarian cancers which have become resistant to the platinum drugs.
Platinum drugs are the standard of care ovarian cancers and most other drugs do not work very well once ovarian cancers have become resistant to carboplatin or cisplatin (platinum resistant ovarian cancers).
So it is very good news that we may have a new option now for this group of patients with platinum resistant ovarian cancers.
Early phase drug trial results of Rela in 2023 were promising but not definitive.
Advanced phase 3 trials of Rela have been positive in improving survival (2025).
The study results were due to presented at the prestigious annual meeting of the American Society of Clinical Oncology (ASCO) at Chicago.( June 2025).
Dr Sundar is a member of American Society of Clinical Oncology (ASCO) and has been a member for more than 25 years.
References
1. Relacorilant + Nab-Paclitaxel in Patients With Recurrent, Platinum-Resistant Ovarian Cancer: A Three-Arm, Randomized, Controlled, Open-Label Phase II Study
Nicoletta Colombo et al. J Clin Oncol. 2023.
2. Targeted oncology. News Article. March 31, 2025. Relacorilant Extends Survival in Platinum-Resistant Ovarian Cancer Mar 31, 2025 . By Jordyn Sava. Fact checked by: Jason M. Broderick
3. ROSELLA: A phase 3 study of relacorilant in combination with nab-paclitaxel versus nab-paclitaxel monotherapy in patients with platinum-resistant ovarian cancer (GOG-3073, ENGOT-ov72).
Disclaimer: Please note – This blog is NOT medical advice. This blog is NOT a expert medical opinion on various topics. This blog is purely for information research only and do check the sources where cited. Please DO consult your own doctor to discuss concerns and options, which are relevant and specific to you. The views expressed in this blog are NOT, in any way whatsoever, intended to be a substitute for professional advice. The blog is NOT previewed, commissioned or otherwise endorsed, in any way, by any organisation that the author is associated with. The views expressed in this blog likely represents some of the author’s personal views held at the time of drafting the blog and MAY CHANGE overtime, particularly when new evidence comes to light.