Advanced bowel cancer is usually treated with chemotherapy.
A subset of bowel cancers carry a genetic change called BRAF V600E mutation.
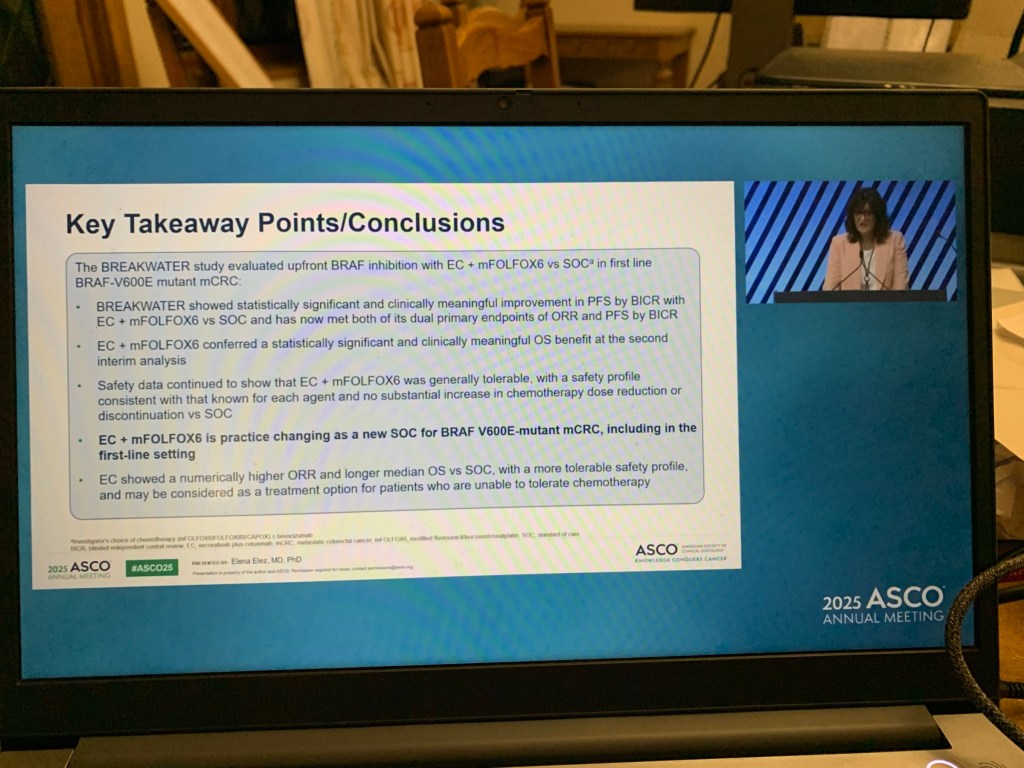
These patients benefit from addition of a drug called Encorafenib to the chemotherapy drugs.
This treatment regimen will become the standard of care for this sub-group of patients.
The study data was presented at the prestigious annual meeting of the American Society of Clinical Oncology (ASCO) at Chicago.
The results were also published in the prestigious NEJM New England Journal of Medicine.
Dr Sundar is a member of American Society of Clinical Oncology (ASCO) and has been a member for more than 25 years.
References
- ASCO. First-line encorafenib + cetuximab + mFOLFOX6 in BRAF V600E-mutant metastatic colorectal cancer (BREAKWATER): Progression-free survival and updated overall survival analyses.
- NEJM. Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer
Authors: Elena Elez, M.D., Ph.D., Takayuki Yoshino, M.D., Ph.D., Lin Shen, M.D., Sara Lonardi, M.D., Eric Van Cutsem, M.D., Ph.D., Cathy Eng, M.D., Tae Won Kim, M.D., Ph.D., +13 , for the BREAKWATER Trial Investigators*Author Info & Affiliations
Published May 30, 2025
DOI: 10.1056/NEJMoa2501912
Copyright © 2025

Disclaimer: Please note – This blog is NOT medical advice. This blog is NOT a expert medical opinion on various topics. This blog is purely for information research only and do check the sources where cited. Please DO consult your own doctor to discuss concerns and options, which are relevant and specific to you. The views expressed in this blog are NOT, in any way whatsoever, intended to be a substitute for professional advice. The blog is NOT previewed, commissioned or otherwise endorsed, in any way, by any organisation that the author is associated with. The views expressed in this blog likely represents some of the author’s personal views held at the time of drafting the blog and MAY CHANGE overtime, particularly when new evidence comes to light.
